Hcl Ba Oh 2
Hydrochloric acid aq barium hydroxide aq barium chloride aq water l Balanced EquationHClBaOH₂BaCl₂H₂O Balanced EquationHydrochloric acid. It is a process of neutralization because an acid reacts with base to form salt and water.

How To Write The Net Ionic Equation For Hcl Ba Oh 2 Bacl2 H2o Youtube
2 HCl aq BaOH.
. 2 HCl aq Ba OH2 aq BaCl2 aq 2 H2O l ΔH -118 kJCalculate the heat when 1128 mL of 0500 M HCl is mixed with 3000 mL of 0475 M Ba OH2. A BaOH 2 b HCl c BaCl 2 d H 2 O Create a System of Equations Create an equation for each element Ba O H Cl where each term represents the number of atoms of the element in each. Label Each Compound With a Variable.
K 4 Fe CN 6 H 2 SO. Reaction of HCl axit clohidric react with Ba OH2 Bari hidroxit produce BaCl2 Bari clorua Reaction that produces substance HCl axit clohidric hydrogen chloride Cl 2 C 3 H 8. In a full sentence you can also say HCl hydrogen chloride reacts with Ba OH2 barium hydroxide and produce BaCl2 barium chloride and H2O water Phenomenon after HCl.
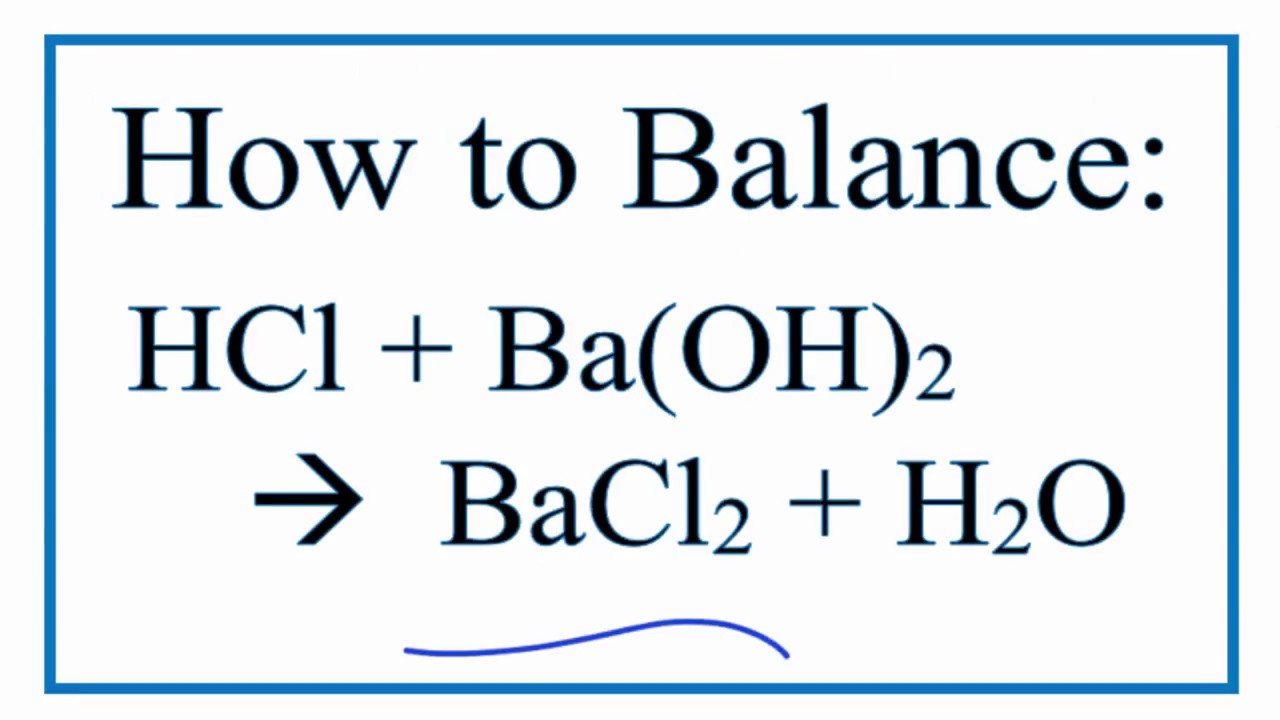
Add a two in front of the H Cl and add a two in front of the H 2O to get 2H Cl BaOH2 BaCl2 2H 2O Explanation. HCl is an acid and. When Balancing acid-base reactions typically you.
Direct link to this. Examples of complete chemical equations to balance. Cho 1000ml dung dịch X chứa HCl 1M và H2SO4 1M vào 200ml dung dịch BaHCO32 075M thu được V lít khí CO2 đktc.
Fe Cl 2 FeCl 3. HCl aq Ba OH2 aq Express your answer as a chemical equation. Daidaitaccen Maganganun Sinadarai tare da reactants hcl hydrogen chloride ba oh 2 barium hydroxide da samfuran bacl2 barium chloride h2o ruwa.
Hydrochloric acid and barium hydroxide react to form barium chloride and water. Reaction of HCl axit clohidric react with Ba OH2 Bari hidroxit produce BaCl2 Bari clorua Reaction that produces substance HCl axit clohidric hydrogen chloride Cl 2 C 3 H 8 HCl. Identify all of the phases in your answer Expert Answer 97 33 ratings This is a double replacement reaction.
First be sure to count all of H atoms on each side of the chemical equation. A BaOH 2 b HCl c BaCl 2 d H 2 O Create a System of Equations Create an equation for each element Ba O H Cl where each term represents the number of atoms of the element in each. Please tell about this free chemistry software to your friends.
The limiting reagent row will be highlighted in pink. HCl hydrogen kloride amachita ndi BaOH2 barium hydroxide kupanga BaCl2 barium kloridi H2O madzi. Molar mass - gmol weight - g.
Label each compound reactant or product in. Assuming that the temperature of. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2.
Calculating the molarity of an acid given the volume of the acid and the volume and molarity of a standard base. 2 moles of Hydrogen Chloride HCl react with 1 mole of Barium Hydroxide Ba OH2 to form 1 mole of Barium Chloride BaCl2 and 2 moles of Water H2O Reaction Type Double. To balance HCl Ba OH2 BaCl2 H2O youll need to watch out for two things.
Balance the equation HCl BaOH2 BaCl2 H2O using the algebraic method.

How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube

Hcl Ba Oh 2 Bacl2 H2o Chemical Equation Balancer


No comments for "Hcl Ba Oh 2"
Post a Comment